Warehouse Environmental Mapping: Ensuring Compliance & Product Quality
by Keith Sheldon on Nov 6, 2025 10:30:00 AM

Temperature and Relative Humidity control is critical in various industries, especially those dealing with sensitive products like pharmaceuticals, food and beverages, chemicals, electronics, and healthcare. A warehouse serves as a hub for the storage and distribution of goods, so maintaining the right environmental conditions is essential for preserving product integrity and quality. Mapping the warehouse for temperature and relative humidity is a vital process that helps ensure consistency throughout a warehouse, especially in environments where products must be stored at specific ranges.
What Is Environmental Mapping in Warehouses?
Mapping involves the process of measuring and recording the temperature and relative humidity distribution within a warehouse or storage area over a set period. This process is usually performed using a network of dataloggers that are placed in strategic locations within the warehouse. The goal is to identify variations across different zones, including hot spots, cold spots, high humidity, low humidity and the fluctuations that might be caused by external factors like doors opening or the HVAC system performance.
Mapping involves:
- Strategically setting up dataloggers at multiple points across the warehouse.
- Monitoring conditions over time, especially during critical operational periods (e.g., loading/unloading, during transportation, or when the HVAC system is running).
- Analyzing data to identify areas that deviate from the required ranges.
How Environmental Mapping Protects Product Quality
Product Quality and Integrity
The primary reason for mapping is to ensure that products are stored in conditions that preserve their quality and integrity. In industries like pharmaceuticals, improper storage can result in chemical degradation, reduced efficacy, or even the destruction of the product. Similarly, food products may spoil or become unsafe to consume if they are exposed to temperatures outside their specified range.
By regularly mapping the warehouse’s conditions businesses can ensure their products are stored under optimal conditions, avoiding damage and ensuring customer satisfaction.
Warehouse Mapping for Regulatory Compliance (GMP, HACCP)
Many industries are subject to strict regulatory standards governing the storage of products. For instance:
- Good Manufacturing Practice (GMP) in pharmaceuticals and biotechnology requires controlled storage conditions for drugs and biologics.
- Hazard Analysis and Critical Control Points (HACCP) in food safety also necessitate temperature monitoring to prevent contamination and ensure food safety.
Mapping is often a key part of compliance efforts, as it provides documented proof that products have been stored within the prescribed limits. Failure to maintain these records can result in fines, regulatory sanctions, or loss of certifications or recalls which can severely impact business operations.
Optimization of Warehouse Operations
Mapping can also provide valuable insights into how efficiently the warehouse environment is being managed. For instance:
- Identifying inconsistencies can help pinpoint inefficiencies in the HVAC system, insulation, or airflow distribution. This can lead to more precise adjustments and system upgrades, ultimately optimizing energy consumption and operational costs.
- Detecting hot or cold spots can lead to better placement of temperature-sensitive goods and ensure that areas of the warehouse that require stricter control are appropriately monitored and managed.
- Monitoring fluctuations can also assist in identifying potential risks from external factors, such as the opening of doors or loading docks.
Optimizing conditions leads to better overall operational efficiency, saving costs, reducing waste, and ensuring that products are stored correctly throughout their life cycle.
Prevention of Losses and Waste
Improper storage can lead to significant losses in both inventory and revenue. For instance, an excursion can ruin batches of pharmaceuticals, resulting in costly product recalls or disposal of goods. Similarly, the spoilage of food items due to inadequate refrigeration can result in waste and financial loss.
By performing environmental mapping regularly, businesses can reduce the chances of excursions, identify problem areas, and prevent potential losses. Additionally, early detection of issues allows for timely interventions, reducing the likelihood of irreversible product damage.
How to Conduct Environmental Mapping in a Warehouse
- Set Clear Objectives
Understand the storage requirements for the specific products. Determine the range required and what areas need to be mapped (e.g., storage shelves, ambient spaces, refrigerated zones, etc.). - Select Data Loggers
Choose the right type of data logger that suits the needs of your warehouse. Wireless sensors are ideal for large spaces, while wired data loggers can be used for more localized temperature monitoring. - Place Sensors Strategically
Place loggers at various points within the warehouse to get a representative sample of variations. Consider areas near HVAC vents, doors, or where products with varying temperature requirements are stored. Also consider where your already installed monitoring and controls sensors are located as well, placing data loggers near these sensors will confirm that your daily operations and data trending are accurate year round and not just during the mapping cycle. - Monitor Over Time
Record temperature data over a reasonable period, including seasonal variations, different operational hours, and various external factors that might influence internal temperatures. - Analyze Results and Implement Changes
After collecting the data, analyze the results for any fluctuations or outliers. Adjust the HVAC system, placement of products or sensors, or warehouse layout to optimize environmental conditions. - Regular Re-Mapping
Environmental Mapping should not be a one-time event. Regular re-mapping ensures that changes in the warehouse environment, system upgrades, or seasonal shifts are accounted for.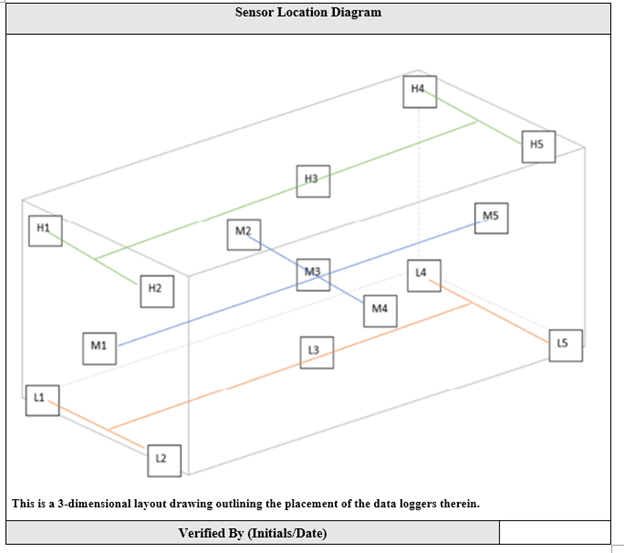
Frequently Asked Questions
Q: How often should warehouses perform environmental mapping?
A: Warehouses should be mapped twice during initial qualification, once in the winter and once in the summer. After the initial year, mapping could be subjected to any major changes made, such as changes to layout of racking, or HVAC changes that result in equipment repairs or replacement. A robust environmental monitoring system that has been tested and providing consistent data can limit the need for frequent remapping of the warehouse spaces.
Q: What tools are best for warehouse temperature mapping?
A: Wireless calibrated data loggers for temperature and humidity with corresponding data logging software are the only tools you need. Your software should be capable of determining the min, max and average readings.
Q: Is environmental mapping required for GMP compliance?
A: Yes. FDA 21 CFR Part 211 requires the proper storage of drug products under labeled conditions. Environmental mapping of storage areas that contain temperature and humidity sensitive products such as pharmaceuticals, biologics or medical devices demonstrates that your warehouse is consistently able to maintain the acceptable conditions of the products that are being stored within it.
Conclusion
Environmental mapping is a crucial process for businesses that store temperature-sensitive products in warehouses. It ensures compliance with industry regulations, protects the quality of stored products, prevents waste, and improves operational efficiency. By conducting thorough and regular mapping exercises, businesses can mitigate risks and maintain compliance. Environmental mapping isn’t just a technical requirement; it’s a critical practice for maintaining the integrity of the entire supply chain.
About the Author
Keith Sheldon is the Commissioning Manager for Hallam-ICS. Keith started his Hallam career in the Hallam-ICS Vermont office and now works out of both the Hallam-ICS Massachusetts and Connecticut offices. Keith has a Bachelor’s of Science degree in Engineering and Management from Clarkson University, and holds a certificate in project management from Worchester Polytechnic Institute. Keith is a member of the ISPE International Society of Pharmaceutical Engineers, and has performed commissioning and qualification projects in the pharmaceutical industry for over 15 years.
Read My Hallam Story
About Hallam-ICS
Hallam-ICS is an engineering and automation company that designs MEP systems for facilities and plants, engineers control and automation solutions, and ensures safety and regulatory compliance through arc flash studies, commissioning, and validation. Our offices are located in Massachusetts, Connecticut, New York, Vermont and North Carolina, Texas and Florida and our projects take us world-wide.
You May Also Like
These Related Stories
CODES: Engineering a Great Customer Experience

Engineering Ethics and Competency



No Comments Yet
Let us know what you think