LIFE SCIENCES
ENGINEERING SOLUTIONS FOR SUCCESS IN LIFE SCIENCES
When precision and compliance are non-negotiable, you need a partner who understands the critical balance between innovation and regulation. Whether you are engaged in benchtop research, complex manufacturing, or anywhere in between, you require engineered solutions that meet stringent regulatory standards and deliver reliable, repeatable results. With over 35 years of experience in GxP environments, our team provides the expertise, timeline management, and integration services necessary to safeguard product quality, patient safety, and data integrity, allowing you to focus on advancing medical breakthroughs.
Years Serving Life Sciences Clients
Life Sciences Clients
Projects Completed or In-Progress
SPECIALTIES
FDA-Regulated Facilities
cGMP Facilities
GMP Validation Areas + Systems
Environmental Monitoring Systems
21 CFR Part 11 Compliant Process Control Systems
Bio-Safety + Cleanroom R&D Facilities
Hazardous Locations
Combustible Dust Locations
Process + Central Plant Utility Design
Accelerate your medical innovation with streamlined processes.
SOLUTIONS FOR LIFE SCIENCES
Our team of engineers, control system integrators, instrumentation technicians, commissioning and validation experts, and arc flash electrical safety specialists have over three decades of Life Science expertise. With a practical understanding of regulatory standards and a focus on the big picture, we’ll ensure that your operation is not only compliant, but more importantly: safe.
MECHANICAL, ELECTRICAL + PROCESS ENGINEERING
MECHANICAL, ELECTRICAL + PROCESS ENGINEERING
PROCESS CONTROL SYSTEMS
PROCESS CONTROL SYSTEMS
MAIN INSTRUMENT VENDOR
MAIN INSTRUMENT VENDOR
COMMISSIONING, VALIDATION + QUALIFICATION
COMMISSIONING, VALIDATION + QUALIFICATION
MECHANICAL, ELECTRICAL + PROCESS ENGINEERING
We provide precision engineering and project management on every project. Our teams can work alongside other experts for a single system or lead your engineering and process solutions end-to-end, from initial design through operational lifecycle. Our engineering expertise are focused on Good Manufacturing Process (GMP) validation areas, Bio-Safety research settings, cleanrooms, vivarium, and hazardous locations including hazardous dust.

PROCESS CONTROL SYSTEMS
Often called on to solve the most complicated challenges in a facility, Hallam-ICS is known for breaking down the barriers to improvement and success – we can design, build, and program your automation system from start to finish. Our strengths within process controls and plant automation include Batch/S88, facility management, and Plant PAx system.

MAIN INSTRUMENT VENDOR
Our unique combination of engineering, automation, installation, and validation experience provides us with a deep understanding of the needs of our clients from instrument specification through bench testing, installation, field calibration and Turnover Package (ETOP) development.
From procurement and receiving to final delivery, we have the personnel power, cash flow, infrastructure and procedures in place in order to handle a vast project. By engaging Hallam-ICS as a Main Instrument Vendor (MIV) early in the project clients have the advantage of avoiding delays and unnecessary errors in order to stay on track and on budget.
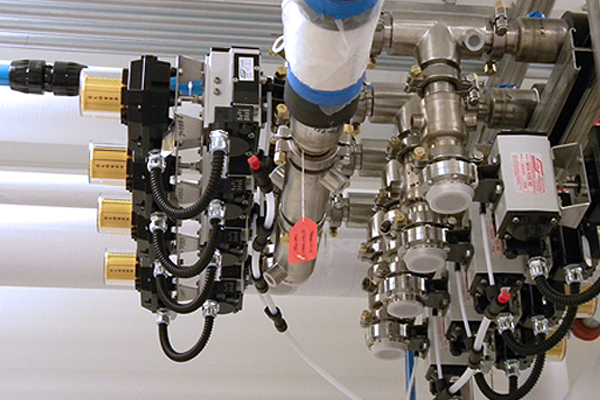
COMMISSIONING, VALIDATION + QUALIFICATION
Our commissioning and validation support Good Manufacturing Practice (GMP) settings, and ensure documented evidence that equipment, software, and instruments used in the manufacture of pharmaceuticals, biologics, and medical devices are fit for use and will provide repeatable functionality.

SUCCESS WITH HALLAM-ICS
Life sciences clients benefit from our engineering expertise, responsiveness, and project management.

CASE STUDY
New Clinical Manufacturing Facility

CASE STUDY
Project Inspire – Durham Clinical Manufacturing Facility
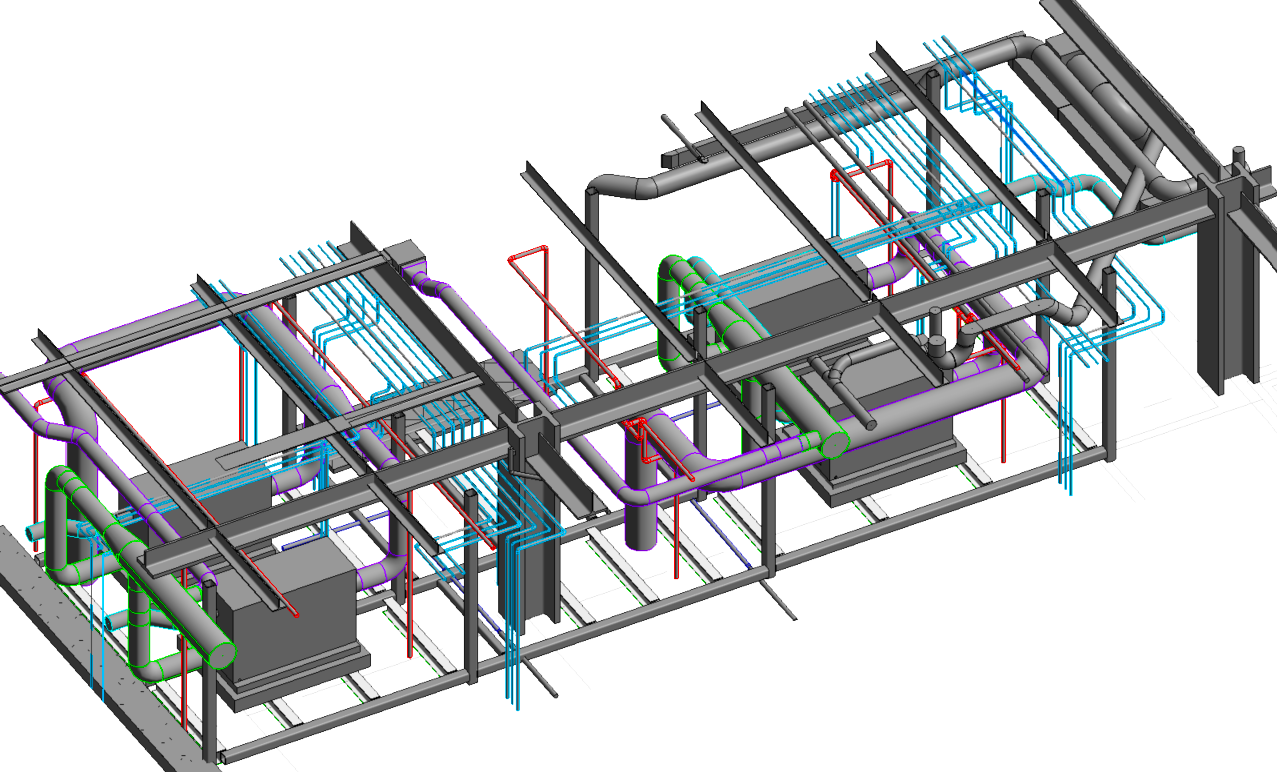
CASE STUDY
Chiller Upgrade Engineering Design
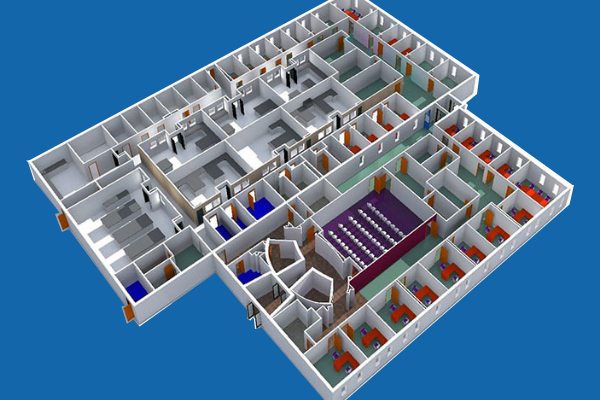
CASE STUDY
New Laboratory Engineering Design

CASE STUDY
B156A Chemistry Labs MEP Engineering Design

CASE STUDY
Siemens Medical Solutions Diagnostics
CASE STUDY
New Manufacturing Facility Instrument Calibration
RELATED RESOURCES
Explore our library of resources to learn more about our exceptional solutions for life science applications.
OUR TAILORED APPROACH
When the health and safety of individuals is at stake, you need a partner who understands the nuances of your industry. At Hallam-ICS, our tailored approach to the life science industry is based on over 35 years of providing engineering, control systems, and validation solutions that solve the most complex challenges in labs and production facilities. We listen to your goals and come to the table with expertise to engineer a timely solution that safeguards product quality, patient safety, and data integrity. Whether you are engaged in benchtop research or complex manufacturing, our team is equipped to support your facility at every stage, from inception to production.
We follow our proprietary CODES™ process — COMMUNICATIONS, OVERSIGHT, DOCUMENTATION, EXPECTATIONS, and SCHEDULE — to deliver exceptional project quality and consistency. Each project starts with a comprehensive discovery phase, when we gather critical information about your needs and milestones to develop a customized project plan that ensures seamless execution. Throughout the project, we maintain open lines of communication and provide detailed documentation, ensuring that every phase is meticulously managed. Our commitment to technical excellence, combined with our robust customer service, guarantees a successful project outcome and an exceptional customer experience.
FROM OUR BLOG
Stay updated with the latest insights, trends, and expert engineering advice on our blog.

Validation in Life Sciences Projects: Ensuring Compliance and Safety

Cleanroom Commissioning: Ensuring Precision, Performance, & Compliance



